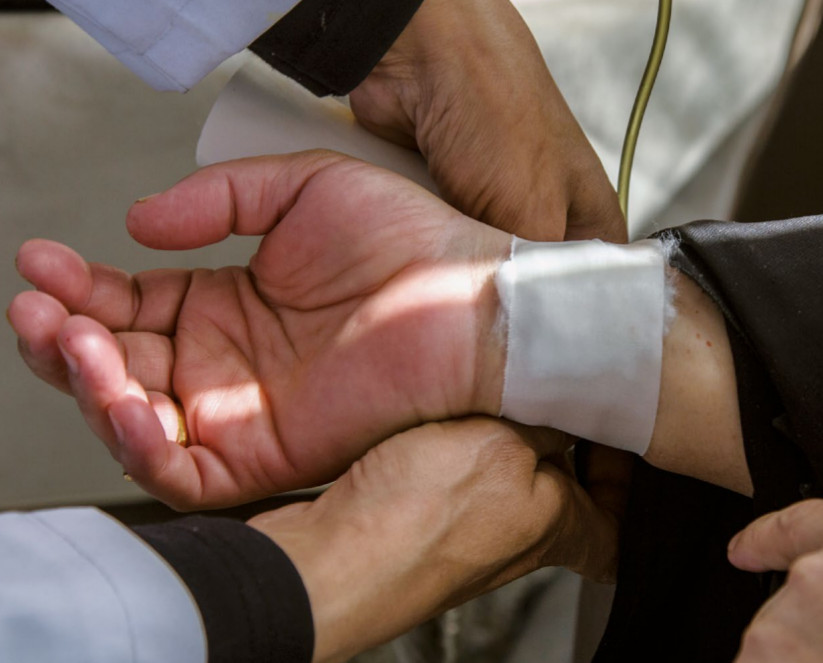
Industry-sponsored clinical drug trials in Egypt
Ethical questions in a challenging context

When engaging in clinical trials in low- and middle-income countries with limited access to treatment, pharmaceutical companies should ascertain that the safety and rights of participants are properly protected and that their practice are in line with the highest ethical standards. They should also make sure that the medications tested in those countries are available at an affordable price. This study brings new evidence that unethical practices occur in TNC sponsored clinical trials conducted in Egypt, and that drugs tested in Egypt are not systematically available to and mostly unaffordable for the Egyptian population.
Meer informatie nodig?
-

Irene Schipper
Senior Onderzoeker
Partners
Publicatie

Related news
-
Datahonger wint het van medisch beroepsgeheim in Europese wet voor gezondheidsdataGeplaatst in categorie:Opinie
 Irene SchipperGepubliceerd op:
Irene SchipperGepubliceerd op: Irene Schipper
Irene Schipper -
Maatschappelijke organisaties roepen de EU op om de belangen van patiënten en burgers centraal te stellen in de European Health Data SpaceGeplaatst in categorie:Gepubliceerd op:Verklaring
-
Bij kankermedicijn Trodelvy is alles gericht op de aandeelhoudersGepubliceerd op:Geplaatst in categorie:Opinie

